



Published: 8/26/2011
 By Vik Kirsch, Mercury staff, www.guelphmercury.com, Updated: August 26, 2011 6:30 AM
By Vik Kirsch, Mercury staff, www.guelphmercury.com, Updated: August 26, 2011 6:30 AM
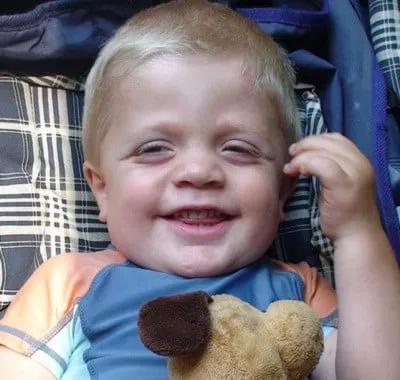
Jasper More will begin receiving life-saving but extremely expensive drug treatment within weeks for a rare genetic disorder.
PALMERSTON — Jasper More will begin receiving life-saving but extremely expensive drug treatment within weeks for a rare genetic disorder.
The Ontario Ministry of Health will fund the enzyme replacement therapy, which can cost up to $800,000 a year, for the 2 ½-year-old Palmerston boy who has Maroteaux-Lamy Syndrome (MPS Type VI). He was diagnosed in the spring.
He is the second Ontario child to receive treatment for the rare disease, which afflicts eight Canadians and roughly 1,100 people globally.
His parents, Darren and Pam More, feel enormous relief at news that the drug regimen is to begin as early as September.
“We’re a lot better than we were in April let me tell you,” Darren More said Wednesday. “In April, when we found out, we were just completely wrecked.”
MPS Type VI is the rarest in a family of diseases that fall under the umbrella of mucopolysaccharidosis disease, a metabolic disorder in which enzymes the body needs to break down sugar carbohydrates are missing or malfunctioning, compromising a child’s development as waste material builds up in the body.
There’s no known cure and the prognosis is a shortened lifespan. But with groundbreaking enzyme replacement therapy, patients can live nearly normal lives, advocates say.
Health Minister Deb Matthews said Wednesday the government is covering the cost after an evaluation by the executive officer of the provincial drug program, who looked at a variety of factors such as the therapy itself and assessments by other jurisdictions, and a rare diseases review committee.
Absolute conclusions, Matthews said, are difficult in cases of ultra-rare illnesses such as Jasper’s form of MPS, but she was happy with the funding decision.
“I’m very pleased,” she said. “It’s the right thing to do.”
The Mores have been helped in learning about MPS and appeals to the government by the Canadian MPS Society and the Isaac Foundation, a Campbellford, Ont. research funder founded by the parents of Isaac McFadyen.
Andrew McFadyen said his seven-year-old son, who has received government-supported therapy since 2006, is doing extremely well.
“(He’s) probably the healthiest he’s ever been. Essentially, we’ve stabilized the disease.”
McFadyen termed the treatment a godsend. “It saved his life, for sure.”
The foundation is funding research into treatments with an ultimate goal of a cure. It’s about to release findings on a research program at a New York hospital on MPS Type VI.
“That’s what Jasper and Isaac both have,” McFadyen said, stressing any advances made will have positive implications for all versions of MPS.
Jasper’s April diagnosis was confirmed through further testing in July, said his father, a purchasing manager for an Arthur-area firm. The couple has two other children, Daphnie, 9, and Clayton, 5, who are free of the disease.
More said when both parents are carriers, as they are, a child has a one-in-four chance of getting the genetic disease. “We had absolutely no idea we were carriers at all,” he recalled.
Jasper was born with two hernias and later developed a growth on his spine. Today, the boy has some enlarged organs and bone deformities, but the condition will improve with therapy. “Type VI is treatable. (It’s) not curable, but treatable.”
The enzyme replacement therapy offered by a California firm is tailor made, so it can’t be mass produced. “It has to be specific to the patient,” More said.
Jasper will have to travel each week to a London, Ont. hospital for an intravenous drip.
The treatments will last “as long as he lives, or until they find a cure,” More said.